Abstract
Purpose
International Prognostic Index (IPS) is the most widely used risk stratification index for advanced stage Hodgkin's lymphoma (HL). The use of (18F)-fluorodeoxyglucose PET/CT at diagnosis allows a better characterization of extra-nodal involvement (ENI). We investigated if the type of ENI could affect the prognosis of stage IV HL patients diagnosed with PET/CT and if a specific prognostic index could be defined for these patients (pts).
Patients and methods
We retrospectively analyzed 220 stage IV HL patients treated from 2005 to 2015 in three LYSA centers. We considered the local investigator interpretation based on the nuclear medicine physician PET/CT report. Regarding ENI, six subgroups were identified: involvement of lung and/or pericard and/or pleural, liver, diffuse and/or focal bone involvement, digestive system, and other involvements; we also considered bone marrow involvement based on the results of bone marrow biopsy. The main outcome was event free survival (EFS) defined by relapse, progression, death from any cause and initiation of a new therapy. For prognostication, we first evaluated the six variables of IPS-6 (corresponding to IPS without "stage IV" item) in this population. ENI was tested adjusted on the retained IPS variables. Univariate and multivariate Cox models were used to assess their prognostic ability for EFS. Cross-validation (10-fold) was used to select the more robust variables avoiding optimism. The finally selected variables constituted a score that was tested on overall survival (OS).
Results
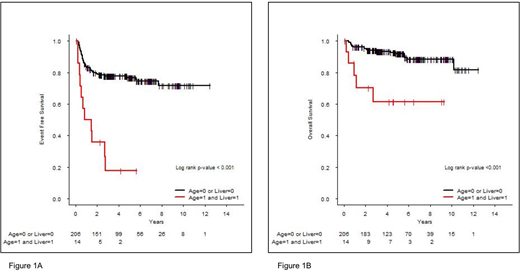
Among the 220 stage IV patients, 135 (61%) were male. Median age was 33 years (range, 16-86) and 72 pts (33%) were ≥45 years. 130 pts (59%) presented constitutional symptoms. Nodular sclerosis subtype was observed in 163 pts (74%), mixed cellularity subtype in 25 pts (11%) and 47/157 pts (30%) presented EBV-positive HL. For biological parameters of IPS, 158 pts (80%) had low albumin level <4g/dL, 66 pts (30%) hemoglobin values <10.5g/dL, white blood cell (WBC) count was >15G/L in 42pts (19%) and lymphocyte count <600/mm3 in 75 pts (34%). The IPS-6 score was 0-2 in 93 pts (47%) and ≥3 in 104 pts (53%). ENI distribution according to PET/CT was: diffuse and/or focal bone involvement (155 pts, 71%), lung-pericard-pleural (94 pts, 43%), liver (37pts, 17%), digestive system (11 pts, 5%), 38 pts (17%) had other ENI; bone marrow involvement according to biopsy concerned 40 pts (21%). Only 1 extra-nodal site was involved in 49% of pts, 2 sites in 33%, 3 sites in 16% and 4 sites in 2%. With a median follow-up of 4.8 years, the 5-year EFS and OS rates were 73% and 89.9%, respectively for the whole cohort. The IPS-6 remained a strong prognostic index in our cohort. Patients with an IPS-0-2 and 3-6 had a 5-year EFS rate of 81.8% and 64% (p=0.008), respectively. The evaluation in univariate analysis of the prognostic value of each individual variable of IPS showed that only age influenced EFS (p=0.002) but albumin level (p=0.92), hemoglobin level (p=0.28), lymphocyte count (p=0.16), WBC count (p=0.10) and sex (p=0.21) had no significant prognostic effect. Regarding ENI, all 6 subgroups were studied: liver involvement was the only extra-nodal site with prognostic impact (HR=1.67 [0.92-3.04], p=0.093) in univariate analysis. We then performed a multivariate analysis integrating age and liver involvement: age was significantly associated with EFS (HR=2.20 [1.32-3.65], p=0.002); liver involvement also presented an influence on EFS (HR=1.72 [0.94-3.13], p=0.076). Thus, we developed a prognostic index with these two variables that defined two distinct risk groups: low risk (age <45 years or no liver involvement, N=206 pts, 94%) and high risk (age ≥45 years and liver involvement, N=14 pts, 6%) (HR=5.09 [2.64-9.85], p<10-3). 5-year EFS rates were respectively 76.8% and 17.9% (Figure 1A). This model also influenced OS with a 5-year OS rate of 91.8% and 61.4% for low and high risk groups, respectively (Figure 1B).
Conclusions:
For stage IV HL defined by PET/CT, we developed a simple prognostic score based on age (≥45y) and liver involvement that identify a subgroup of patients with a poor outcome. These findings need to be validated in independent cohorts. Based on these results, whether HL pathogenesis differs by ENI sites should be investigated.
Bachy:Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; Amgen: Honoraria. Karlin:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support. Sarkozy:ROCHE: Consultancy. Traverse-Glehen:Takeda: Research Funding; Astra Zeneca: Other: Travel. Salles:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Servier: Honoraria, Other: Advisory Board; Novartis: Consultancy, Honoraria; BMS: Honoraria, Other: Advisory Board; Morphosys: Honoraria; Janssen: Honoraria, Other: Advisory Board; Abbvie: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Celgene: Honoraria, Other: Advisory Board, Research Funding; Epizyme: Honoraria; Gilead: Honoraria, Other: Advisory Board; Acerta: Honoraria; Merck: Honoraria; Servier: Honoraria; Takeda: Honoraria. Casasnovas:Takeda: Consultancy; Gilead: Consultancy, Research Funding; AbbVie: Consultancy; Roche: Consultancy, Research Funding; Bristol-Meyers Squibb: Consultancy; Merck: Consultancy. Ghesquieres:Celgene: Consultancy; Gilead: Consultancy; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.